WO2002000022A1 - Composition for inhibiting feeding of weevils - Google Patents
Composition for inhibiting feeding of weevils Download PDFInfo
- Publication number
- WO2002000022A1 WO2002000022A1 PCT/SE2001/001458 SE0101458W WO0200022A1 WO 2002000022 A1 WO2002000022 A1 WO 2002000022A1 SE 0101458 W SE0101458 W SE 0101458W WO 0200022 A1 WO0200022 A1 WO 0200022A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- methyl
- compound
- composition
- active compound
- present
- Prior art date
Links
- 0 *C(NO)=Cc1c(*)cc(*)cc1 Chemical compound *C(NO)=Cc1c(*)cc(*)cc1 0.000 description 1
- XVOHPSXSOVMGCY-UHFFFAOYSA-N CCCCCC(C)(C(CC=C1C)CC1=O)O Chemical compound CCCCCC(C)(C(CC=C1C)CC1=O)O XVOHPSXSOVMGCY-UHFFFAOYSA-N 0.000 description 1
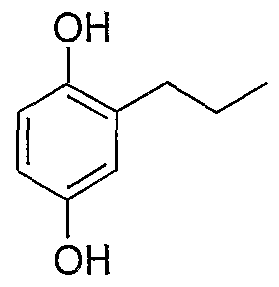
- NJRNUAVVFBHIPT-UHFFFAOYSA-N CCCc1cc(O)ccc1O Chemical compound CCCc1cc(O)ccc1O NJRNUAVVFBHIPT-UHFFFAOYSA-N 0.000 description 1
- PKZJLOCLABXVMC-UHFFFAOYSA-N COc1c(C=O)cccc1 Chemical compound COc1c(C=O)cccc1 PKZJLOCLABXVMC-UHFFFAOYSA-N 0.000 description 1
- ZRSNZINYAWTAHE-UHFFFAOYSA-N COc1ccc(C=O)cc1 Chemical compound COc1ccc(C=O)cc1 ZRSNZINYAWTAHE-UHFFFAOYSA-N 0.000 description 1
- WMPDAIZRQDCGFH-UHFFFAOYSA-N COc1cccc(C=O)c1 Chemical compound COc1cccc(C=O)c1 WMPDAIZRQDCGFH-UHFFFAOYSA-N 0.000 description 1
- KKVZAVRSVHUSPL-GQCTYLIASA-N COc1ccccc1/C=C/C=O Chemical compound COc1ccccc1/C=C/C=O KKVZAVRSVHUSPL-GQCTYLIASA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C49/00—Ketones; Ketenes; Dimeric ketenes; Ketonic chelates
- C07C49/587—Unsaturated compounds containing a keto groups being part of a ring
- C07C49/703—Unsaturated compounds containing a keto groups being part of a ring containing hydroxy groups
- C07C49/713—Unsaturated compounds containing a keto groups being part of a ring containing hydroxy groups a keto group being part of a six-membered ring
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N25/00—Biocides, pest repellants or attractants, or plant growth regulators, characterised by their forms, or by their non-active ingredients or by their methods of application, e.g. seed treatment or sequential application; Substances for reducing the noxious effect of the active ingredients to organisms other than pests
- A01N25/08—Biocides, pest repellants or attractants, or plant growth regulators, characterised by their forms, or by their non-active ingredients or by their methods of application, e.g. seed treatment or sequential application; Substances for reducing the noxious effect of the active ingredients to organisms other than pests containing solids as carriers or diluents
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N25/00—Biocides, pest repellants or attractants, or plant growth regulators, characterised by their forms, or by their non-active ingredients or by their methods of application, e.g. seed treatment or sequential application; Substances for reducing the noxious effect of the active ingredients to organisms other than pests
- A01N25/08—Biocides, pest repellants or attractants, or plant growth regulators, characterised by their forms, or by their non-active ingredients or by their methods of application, e.g. seed treatment or sequential application; Substances for reducing the noxious effect of the active ingredients to organisms other than pests containing solids as carriers or diluents
- A01N25/10—Macromolecular compounds
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N25/00—Biocides, pest repellants or attractants, or plant growth regulators, characterised by their forms, or by their non-active ingredients or by their methods of application, e.g. seed treatment or sequential application; Substances for reducing the noxious effect of the active ingredients to organisms other than pests
- A01N25/24—Biocides, pest repellants or attractants, or plant growth regulators, characterised by their forms, or by their non-active ingredients or by their methods of application, e.g. seed treatment or sequential application; Substances for reducing the noxious effect of the active ingredients to organisms other than pests containing ingredients to enhance the sticking of the active ingredients
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N25/00—Biocides, pest repellants or attractants, or plant growth regulators, characterised by their forms, or by their non-active ingredients or by their methods of application, e.g. seed treatment or sequential application; Substances for reducing the noxious effect of the active ingredients to organisms other than pests
- A01N25/26—Biocides, pest repellants or attractants, or plant growth regulators, characterised by their forms, or by their non-active ingredients or by their methods of application, e.g. seed treatment or sequential application; Substances for reducing the noxious effect of the active ingredients to organisms other than pests in coated particulate form
- A01N25/28—Microcapsules or nanocapsules
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N25/00—Biocides, pest repellants or attractants, or plant growth regulators, characterised by their forms, or by their non-active ingredients or by their methods of application, e.g. seed treatment or sequential application; Substances for reducing the noxious effect of the active ingredients to organisms other than pests
- A01N25/34—Shaped forms, e.g. sheets, not provided for in any other sub-group of this main group
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N31/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic oxygen or sulfur compounds
- A01N31/08—Oxygen or sulfur directly attached to an aromatic ring system
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N31/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic oxygen or sulfur compounds
- A01N31/08—Oxygen or sulfur directly attached to an aromatic ring system
- A01N31/16—Oxygen or sulfur directly attached to an aromatic ring system with two or more oxygen or sulfur atoms directly attached to the same aromatic ring system
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N35/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having two bonds to hetero atoms with at the most one bond to halogen, e.g. aldehyde radical
- A01N35/02—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having two bonds to hetero atoms with at the most one bond to halogen, e.g. aldehyde radical containing aliphatically bound aldehyde or keto groups, or thio analogues thereof; Derivatives thereof, e.g. acetals
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N35/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having two bonds to hetero atoms with at the most one bond to halogen, e.g. aldehyde radical
- A01N35/04—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having two bonds to hetero atoms with at the most one bond to halogen, e.g. aldehyde radical containing aldehyde or keto groups, or thio analogues thereof, directly attached to an aromatic ring system, e.g. acetophenone; Derivatives thereof, e.g. acetals
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N35/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having two bonds to hetero atoms with at the most one bond to halogen, e.g. aldehyde radical
- A01N35/06—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having two bonds to hetero atoms with at the most one bond to halogen, e.g. aldehyde radical containing keto or thioketo groups as part of a ring, e.g. cyclohexanone, quinone; Derivatives thereof, e.g. ketals
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/02—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms
- A01N43/04—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom
- A01N43/06—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom five-membered rings
- A01N43/08—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom five-membered rings with oxygen as the ring hetero atom
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/02—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms
- A01N43/04—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom
- A01N43/20—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom three- or four-membered rings
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/02—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms
- A01N43/24—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with two or more hetero atoms
- A01N43/26—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with two or more hetero atoms five-membered rings
- A01N43/28—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with two or more hetero atoms five-membered rings with two hetero atoms in positions 1,3
- A01N43/30—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with two or more hetero atoms five-membered rings with two hetero atoms in positions 1,3 with two oxygen atoms in positions 1,3, condensed with a carbocyclic ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C45/00—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds
- C07C45/61—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds by reactions not involving the formation of >C = O groups
- C07C45/67—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds by reactions not involving the formation of >C = O groups by isomerisation; by change of size of the carbon skeleton
- C07C45/68—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds by reactions not involving the formation of >C = O groups by isomerisation; by change of size of the carbon skeleton by increase in the number of carbon atoms
- C07C45/72—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds by reactions not involving the formation of >C = O groups by isomerisation; by change of size of the carbon skeleton by increase in the number of carbon atoms by reaction of compounds containing >C = O groups with the same or other compounds containing >C = O groups
- C07C45/74—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds by reactions not involving the formation of >C = O groups by isomerisation; by change of size of the carbon skeleton by increase in the number of carbon atoms by reaction of compounds containing >C = O groups with the same or other compounds containing >C = O groups combined with dehydration
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C47/00—Compounds having —CHO groups
- C07C47/20—Unsaturated compounds having —CHO groups bound to acyclic carbon atoms
- C07C47/277—Unsaturated compounds having —CHO groups bound to acyclic carbon atoms containing ether groups, groups, groups, or groups
Definitions
- Neem oil is, however, not equally active against all insects and has turned out to have a limited effect against Hylobius abietis and H. pales.
- the gnawing inhibiting compound is present in a bound form to a substance of the group of zeolites, cyclodextrines, homogenous porous microspheres, spherical vesicles, hollow fibres, such as textile fibres (wool) capillaries of synthetic material (polymers), starch, and polymer films, optionally in combination with a carrier and/or adhering agent, whereby the gnawing inhibiting compound is present in an amount of 1 to 10 % by weight.
- a dry round flask (argon atmosphere) comtaining activated chips of magnesium was provided with 31 ml of dry Et 2 O.
- 15 g (78 mmol) of n-octyl bromide (dried K 2 CO 3 , distilled prior to use) were added dropvwise simultaneously with refluxing the mixture gently.
- the Grignard reagens formed was then added dropwise to another round flask (argon atmosphere) containing a stirred solution of 1.1 g (5.8 mmol) of Cul in 77 ml of dry THF at -30 °C followed by addition of 10 g (42 mmol) the silylether-epoxide (prepared as described above).
- the resulting mixture was allowed to reach room temperature during 15 hrs stirring and was quenched by adding 400 ml of NH C1
- (5R)-5-[(lR/S)-l-Hydroxy-l-methyl-decyl]-2-methyl-cyclohex-2-enone Prepared in accordance with the same procedure as (5R)-5-[(lR/S)-l-Hydroxy-l-methyl-hexyl]-2-methyl-cyclohex-2-enone, described above. It was distilled using bulb to bulb distillation, 220 °C (heating chamber)/0.4 mbar. This provided the diastereomers ( ⁇ 1:1 mixture) as a weakly yelow syrup.
- Method B 1 mol of a suitable aromathic aldehyde dissolved in 200 ml of ethanol (95%). 1.25 mol NaOH or KOH dissolved in 250 ml of ethanol and 250 ml of H 2 O were charged to the solution. The solution was cooled -10 °C and 1.4 mol propanal or acetaldehyde, alternatively, were charged slowly at room temperature, during the charging the temperature was never allowed to exceed 20 °C. The solution was allowed to stand at room temperature. The reaction was disrupted by neutralizing the reaction mixture using -200 ml 6 M of HCl and was extracted twice using ether, the organic phases were washed with NaHCO 3s H 2 O and finally with saturated salt solution and was dried using MgSO 4 . The resulting oil or crystals, alternatively, after filtration, was purified by means of distillation and/or chromatography or recrystallization, alternatively.
- CE8 3-(2-Methoxyphenyl)-propanal-2-ene Prepared in accordance with method B using 2- methoxybensaldehyde, NaOH and distilled acetaldehyde. The reaction was disrupted 1 hr after finishing the charging o the acetaldehyde. Crystals having 96% GC-purity were obtained after distillation and MPLC chromatography using ether/pentane (1:9), bp 101 °C/0.5 mbar (litt. 128-130 °C/0.6 mmHg) 4 , mp 43-46 °C (litt. 45-46 °C) 5 .
Abstract
Description
Claims
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PL36050201A PL360502A1 (en) | 2000-06-26 | 2001-06-26 | Composition for inhibiting feeding of weevils |
| EP01944068A EP1294229A1 (en) | 2000-06-26 | 2001-06-26 | Composition for inhibiting feeding of weevils |
| AU66509/01A AU6650901A (en) | 2000-06-26 | 2001-06-26 | Composition for inhibiting feeding of weevils |
| CA002413508A CA2413508A1 (en) | 2000-06-26 | 2001-06-26 | Composition for inhibiting feeding of weevils |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| SE0002385-3 | 2000-06-26 | ||
| SE0002385A SE0002385L (en) | 2000-06-26 | 2000-06-26 | Composition |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2002000022A1 true WO2002000022A1 (en) | 2002-01-03 |
Family
ID=20280237
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/SE2001/001458 WO2002000022A1 (en) | 2000-06-26 | 2001-06-26 | Composition for inhibiting feeding of weevils |
Country Status (6)
| Country | Link |
|---|---|
| EP (1) | EP1294229A1 (en) |
| AU (1) | AU6650901A (en) |
| CA (1) | CA2413508A1 (en) |
| PL (1) | PL360502A1 (en) |
| SE (1) | SE0002385L (en) |
| WO (1) | WO2002000022A1 (en) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2004002224A1 (en) * | 2002-06-28 | 2004-01-08 | Forskarpatent I Syd Ab | Composition for inhibiting gnawing and feeding by insects and animals intended to be applied on seedlings and trees |
| CN109020794A (en) * | 2018-08-27 | 2018-12-18 | 上海华堇生物技术有限责任公司 | The preparation method of 3- methoxycinnamic aldehyde |
| CN109180451A (en) * | 2018-08-27 | 2019-01-11 | 上海华堇生物技术有限责任公司 | The preparation method of 2,3- dimethoxycinnamaldehyde |
| CN113651682A (en) * | 2021-09-23 | 2021-11-16 | 八叶草健康产业研究院(厦门)有限公司 | Synthetic method of 2-methoxycinnamaldehyde |
Citations (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3641680A1 (en) * | 1986-12-05 | 1988-06-16 | Niedersaechsisches Ministerium | Substance for the protection against bark damage caused by the fir-tree weevil (large brown pine weevil; Hylobius abietis) on young woodland plants |
| EP0272374A2 (en) * | 1986-12-19 | 1988-06-29 | S.C. Johnson & Son, Inc. | Bioactive compositions and methods of producing and using same |
| JPS6419011A (en) * | 1987-07-13 | 1989-01-23 | Mitsuo Matsui | Vermin repellent |
| CN1105658A (en) * | 1994-01-22 | 1995-07-26 | 中国科学院昆明植物研究所 | d-8-acyloxy-allodihydro-carvones compounds and synthetic method thereof |
| US5518757A (en) * | 1993-08-31 | 1996-05-21 | The United States Of America As Represented By The Department Of Agriculture | 4-allylanisole analog scolytid repellents |
| WO1997035476A2 (en) * | 1996-03-25 | 1997-10-02 | Proguard, Inc. | Use of aromatic aldehydes as insecticides and for killing arachnids |
| US5695807A (en) * | 1993-08-31 | 1997-12-09 | The United States Of America As Represented By The Secretary Of Agriculture | 4-allylanisole analog scolytid repellents |
| US5780515A (en) * | 1996-03-21 | 1998-07-14 | Rockhurst University | Benzoquinone and hydroquinone derivatives for use as insect feeding deterrents |
| US5792467A (en) * | 1995-06-07 | 1998-08-11 | Proguard, Inc. | Repellent compositions containing aromatic aldehydes |
| EP0903081A1 (en) * | 1997-09-17 | 1999-03-24 | Göldner, Peter, Dipl.-Ing. | Composition for protecting plants against undesired organisms |
| JPH11346635A (en) * | 1998-06-09 | 1999-12-21 | Daiwa Kagaku Kogyo Kk | Repellent composition for rice weevil |
| WO2000013658A1 (en) * | 1998-09-02 | 2000-03-16 | The Procter & Gamble Company | A composition containing insect repellent |
| US6051612A (en) * | 1996-12-09 | 2000-04-18 | Simon Fraser University | Non-host volatiles as repellents for conifer-infesting bark beetles |
| WO2000056152A1 (en) * | 1999-03-23 | 2000-09-28 | Robigus Ab | Use for conifer sapling protection |
| WO2000062611A1 (en) * | 1999-04-19 | 2000-10-26 | Bayer Aktiengesellschaft | Pearl polymer containing agrochemical active substances |
| WO2001033963A1 (en) * | 1999-11-09 | 2001-05-17 | The Procter & Gamble Company | A composition comprising solubilized repellent active component |
| WO2001034213A1 (en) * | 1999-11-09 | 2001-05-17 | The Procter & Gamble Company | Cyclodextrin compositions for odor, insect and dust mite contol |
-
2000
- 2000-06-26 SE SE0002385A patent/SE0002385L/en not_active Application Discontinuation
-
2001
- 2001-06-26 WO PCT/SE2001/001458 patent/WO2002000022A1/en not_active Application Discontinuation
- 2001-06-26 EP EP01944068A patent/EP1294229A1/en not_active Withdrawn
- 2001-06-26 CA CA002413508A patent/CA2413508A1/en not_active Abandoned
- 2001-06-26 AU AU66509/01A patent/AU6650901A/en not_active Abandoned
- 2001-06-26 PL PL36050201A patent/PL360502A1/en not_active Application Discontinuation
Patent Citations (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3641680A1 (en) * | 1986-12-05 | 1988-06-16 | Niedersaechsisches Ministerium | Substance for the protection against bark damage caused by the fir-tree weevil (large brown pine weevil; Hylobius abietis) on young woodland plants |
| EP0272374A2 (en) * | 1986-12-19 | 1988-06-29 | S.C. Johnson & Son, Inc. | Bioactive compositions and methods of producing and using same |
| JPS6419011A (en) * | 1987-07-13 | 1989-01-23 | Mitsuo Matsui | Vermin repellent |
| US5695807A (en) * | 1993-08-31 | 1997-12-09 | The United States Of America As Represented By The Secretary Of Agriculture | 4-allylanisole analog scolytid repellents |
| US5518757A (en) * | 1993-08-31 | 1996-05-21 | The United States Of America As Represented By The Department Of Agriculture | 4-allylanisole analog scolytid repellents |
| CN1105658A (en) * | 1994-01-22 | 1995-07-26 | 中国科学院昆明植物研究所 | d-8-acyloxy-allodihydro-carvones compounds and synthetic method thereof |
| US5792467A (en) * | 1995-06-07 | 1998-08-11 | Proguard, Inc. | Repellent compositions containing aromatic aldehydes |
| US5780515A (en) * | 1996-03-21 | 1998-07-14 | Rockhurst University | Benzoquinone and hydroquinone derivatives for use as insect feeding deterrents |
| WO1997035476A2 (en) * | 1996-03-25 | 1997-10-02 | Proguard, Inc. | Use of aromatic aldehydes as insecticides and for killing arachnids |
| US6051612A (en) * | 1996-12-09 | 2000-04-18 | Simon Fraser University | Non-host volatiles as repellents for conifer-infesting bark beetles |
| EP0903081A1 (en) * | 1997-09-17 | 1999-03-24 | Göldner, Peter, Dipl.-Ing. | Composition for protecting plants against undesired organisms |
| JPH11346635A (en) * | 1998-06-09 | 1999-12-21 | Daiwa Kagaku Kogyo Kk | Repellent composition for rice weevil |
| WO2000013658A1 (en) * | 1998-09-02 | 2000-03-16 | The Procter & Gamble Company | A composition containing insect repellent |
| WO2000056152A1 (en) * | 1999-03-23 | 2000-09-28 | Robigus Ab | Use for conifer sapling protection |
| WO2000062611A1 (en) * | 1999-04-19 | 2000-10-26 | Bayer Aktiengesellschaft | Pearl polymer containing agrochemical active substances |
| WO2001033963A1 (en) * | 1999-11-09 | 2001-05-17 | The Procter & Gamble Company | A composition comprising solubilized repellent active component |
| WO2001034213A1 (en) * | 1999-11-09 | 2001-05-17 | The Procter & Gamble Company | Cyclodextrin compositions for odor, insect and dust mite contol |
Non-Patent Citations (3)
| Title |
|---|
| DATABASE CAPLUS [online] KLEPZIG KIER D. ET AL.: "Laboratory evaluation of plant-derived antifeedants against the pine weevil hylobius abietis (Coleoptera: Curculionidae)", XP002953357, accession no. STN Database accession no. 1999:797200 * |
| DATABASE CAPLUS [online] SUN HANDONG ET AL.: "Preparation of (+)-8-acyloxy-allodihydrocarbones compounds as repellents", XP002953358, accession no. STN Database accession no. 1995:997887 * |
| J. ECON. ENTOMOL., vol. 92, no. 3, 1999, pages 644 - 650 * |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2004002224A1 (en) * | 2002-06-28 | 2004-01-08 | Forskarpatent I Syd Ab | Composition for inhibiting gnawing and feeding by insects and animals intended to be applied on seedlings and trees |
| CN109020794A (en) * | 2018-08-27 | 2018-12-18 | 上海华堇生物技术有限责任公司 | The preparation method of 3- methoxycinnamic aldehyde |
| CN109180451A (en) * | 2018-08-27 | 2019-01-11 | 上海华堇生物技术有限责任公司 | The preparation method of 2,3- dimethoxycinnamaldehyde |
| CN113651682A (en) * | 2021-09-23 | 2021-11-16 | 八叶草健康产业研究院(厦门)有限公司 | Synthetic method of 2-methoxycinnamaldehyde |
Also Published As
| Publication number | Publication date |
|---|---|
| AU6650901A (en) | 2002-01-08 |
| SE0002385L (en) | 2001-12-27 |
| CA2413508A1 (en) | 2002-01-23 |
| EP1294229A1 (en) | 2003-03-26 |
| SE0002385D0 (en) | 2000-06-26 |
| PL360502A1 (en) | 2004-09-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Hall et al. | Identification of components of male-produced pheromone of coffee white stemborer, Xylotrechus quadripes | |
| Lacey et al. | A male-produced aggregation pheromone blend consisting of alkanediols, terpenoids, and an aromatic alcohol from the cerambycid beetle Megacyllene caryae | |
| SU671701A3 (en) | Insecticide | |
| FI56475C (en) | D-CIS TRANSKRYSANTEMAT INNEHAOLLANDE INSEKTICIDKOMPOSITION | |
| IL42406A (en) | 3-methyl-1-oxo-2-(2-propynyl)-2-cyclopenten-4-yl ester of 2,2,3-tri-(or 2,2,3,3-tetra)methylcyclopropanecarboxylic acid,a process for preparing the same and insecticidal compositions containing the same | |
| US3970703A (en) | Phenylacetic acid derivatives | |
| US3864388A (en) | Alkynyl esters of {60 -alkoxy-{60 -phenyl alkenoic acid | |
| Atta et al. | Photoremovable protecting groups as controlled-release device for sex pheromone | |
| US3072526A (en) | Insect lures | |
| RU1811368C (en) | Method of struggle against harmful insects | |
| EP1294229A1 (en) | Composition for inhibiting feeding of weevils | |
| NO129203B (en) | ||
| CN109221119A (en) | It is a kind of for preventing and treating the sex pheromone composition and application of three-spotted plusia | |
| US4877607A (en) | Attractants for Dacus latifrons, the Malaysian fruit fly | |
| De Silva et al. | Identification of sex pheromone components of blueberry spanworm Itame argillacearia (Lepidoptera: Geometridae) | |
| US3515730A (en) | Thenyl esters of cyclopropanecarboxylic acids | |
| 小川邦彦 et al. | Metabolism of 2-sec-Butylphenyl N-Methylcarbamate (Bassa®, BPMC) in Rice Plants and Its Degradation in Soils | |
| US4075320A (en) | Attractant for ants | |
| CA1138467A (en) | Insecticidal esters | |
| EP3882232B1 (en) | Composition attracting the species delottococcus aberiae | |
| US2851392A (en) | Esters of 6-methyl-3-cyclohexene-1-carboxylic acid as attractants for the mediterranean fruit fly | |
| US4632827A (en) | Pest control compositions | |
| CN110754474B (en) | Application of quinidine or quinidine derivatives, botanical pesticide, quinidine derivatives and preparation method thereof | |
| WO2004002224A1 (en) | Composition for inhibiting gnawing and feeding by insects and animals intended to be applied on seedlings and trees | |
| US4563348A (en) | Sec-butyl (Z)-7-tetradecenoate and its use as a sex attractant for the grapeleaf skeletonizer |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AG AL AM AT AT AU AZ BA BB BG BR BY BZ CA CH CN CO CR CU CZ CZ DE DE DK DK DM DZ EC EE EE ES FI FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NO NZ PL PT RO RU SD SE SG SI SK SK SL TJ TM TR TT TZ UA UG US UZ VN YU ZA ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): GH GM KE LS MW MZ SD SL SZ TZ UG ZW AM AZ BY KG KZ MD RU TJ TM AT BE CH CY DE DK ES FI FR GB GR IE IT LU MC NL PT SE TR BF BJ CF CG CI CM GA GN GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| REG | Reference to national code |
Ref country code: DE Ref legal event code: 8642 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2001944068 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2413508 Country of ref document: CA |
|
| WWP | Wipo information: published in national office |
Ref document number: 2001944068 Country of ref document: EP |
|
| NENP | Non-entry into the national phase |
Ref country code: JP |
|
| WWW | Wipo information: withdrawn in national office |
Ref document number: 2001944068 Country of ref document: EP |